How To Draw A Peptide Bond
Peptides & Proteins
1. The Peptide Bail
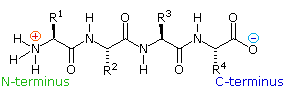
If the amine and carboxylic acid functional groups in amino acids bring together together to course amide bonds, a chain of amino acrid units, chosen a peptide, is formed. A uncomplicated tetrapeptide structure is shown in the post-obit diagram. By convention, the amino acrid component retaining a free amine grouping is drawn at the left stop (the N-terminus) of the peptide chain, and the amino acid retaining a free carboxylic acid is fatigued on the right (the C-terminus). Equally expected, the free amine and carboxylic acid functions on a peptide chain class a zwitterionic structure at their isoelectric pH.
Past clicking the " Grow Peptide " button, an animation showing the associates of this peptide volition be displayed. The " Evidence Construction " button displays some bail angles and lengths that are characteristic of these compounds.
 |
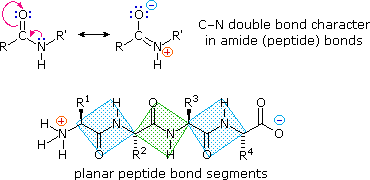
The conformational flexibility of peptide bondage is limited chiefly to rotations about the bonds leading to the alpha-carbon atoms. This restriction is due to the rigid nature of the amide (peptide) bond. As shown in the post-obit diagram, nitrogen electron pair delocalization into the carbonyl group results in pregnant double bond character between the carbonyl carbon and the nitrogen. This keeps the peptide links relatively planar and resistant to conformational change. The color shaded rectangles in the lower structure define these regions, and place the relatively facile rotations that may accept identify where the corners meet (i.e. at the alpha-carbon). This attribute of peptide construction is an of import factor influencing the conformations adopted by proteins and large peptides.
2. The Main Structure of Peptides
Considering the N-terminus of a peptide chain is distinct from the C-terminus, a pocket-size peptide equanimous of different aminoacids may have a several constitutional isomers. 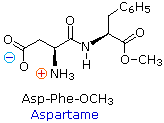 For example, a dipeptide made from 2 different amino acids may have two different structures. Thus, aspartic acid (Asp) and phenylalanine (Phe) may be combined to make Asp-Phe or Phe-Asp, think that the amino acid on the left is the N-terminus. The methyl ester of the first dipeptide (construction on the right) is the bogus sweetener aspartame, which is nearly 200 times sweeter than sucrose. Neither of the component amino acids is sweet (Phe is actually bitter), and derivatives of the other dipeptide (Phe-Asp) are not sweet.
For example, a dipeptide made from 2 different amino acids may have two different structures. Thus, aspartic acid (Asp) and phenylalanine (Phe) may be combined to make Asp-Phe or Phe-Asp, think that the amino acid on the left is the N-terminus. The methyl ester of the first dipeptide (construction on the right) is the bogus sweetener aspartame, which is nearly 200 times sweeter than sucrose. Neither of the component amino acids is sweet (Phe is actually bitter), and derivatives of the other dipeptide (Phe-Asp) are not sweet.
A tripeptide equanimous of iii different amino acids tin can be made in half dozen different constitutions, and the tetrapeptide shown above (composed of four different amino acids) would have 24 constitutional isomers. When all twenty of the natural amino acids are possible components of a peptide, the possible combinations are enormous. Simple statistical probability indicates that the decapeptides fabricated upwardly from all possible combinations of these amino acids would full 20ten!
Natural peptides of varying complexity are abundant. The simple and widely distributed tripeptide glutathione (first entry in the following table), is interesting because the side-concatenation carboxyl part of the N-terminal glutamic acid is used for the peptide bond. An North-terminal glutamic acid may likewise shut to a lactam band, as in the case of TRH (second entry). The abbreviation for this transformed unit is pGlu (or pE), where p stands for "pyro" (such band closures often occur on heating). The larger peptides in the table also demonstrate the importance of amino acrid abbreviations, since a full structural formula for a nonapeptide (or larger) would show to be complex and unwieldy. The formulas using single alphabetic character abbreviations are colored reddish.
The ten peptides listed in this table brand use of all xx common amino acids. Note that the C-terminal unit has the grade of an amide in some cases (e.g. TRH, angiotensin & oxytocin). When two or more cysteines are nowadays in a peptide chain, they are oft joined past disulfide bonds (e.g. oxytocin & endothelin); and in the case of insulin, ii split up peptide bondage (A & B) are held together by such links.
| Name (residues) | Source or Office | Amino Acrid Sequence |
|---|---|---|
| Glutathione (3) | Nearly Living Cells (stimulates tissue growth) | (+)H3NCH(CO2 (–))CHiiCH2CONHCH(CH2SH)CONHCHtwoCOiiH γ-Glu-Cys-Gly (or γECG) |
| TRH (3) | Hypothalmic Neurohormone (governs release of thyrotropin) | 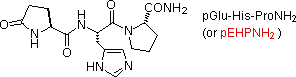 |
| Angiotensin II (8) | Pressor Agent (acts on the adrenal gland) | Asp-Arg-Val-Tyr-Ile-His-Pro-PheNHtwo (or DRVYIHPFNH2 ) |
| Bradykinen (9) | Hypotensive Vasodilator (acts on smooth musculus) | Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg (or RPPGFSPFR) |
| Oxytocin (ix) | Uterus-Contracting Hormone (as well stimulates lactation) |  |
| Somatostatin (14) | Inhibits Growth Hormone Release (used to treat ulcers) |  |
| Endothelin (21) | Potent Vasoconstrictor (structurally similar to some snake venoms) |  |
| Melittin (26) | Dearest Bee Venom (used to treat rheumatism) | Gly-Ile-Gly-Ala-Val-Leu-Lys-Val-Leu-Thr-Thr-Gly-Leu-Pro~ ~Ala-Leu-Ile-Ser-Trp-Ile-Lys-Arg-Lys-Arg-Gln-GlnNH2 (or GIGAVLKVLTTGLPALISWIKRKRQQNH2 ) |
| Glucagon (29) | Hyperglycemic Cistron (used every bit an anti-diabetic) | His-Ser-Gln-Gly-Thr-Phe-Thr-Ser-Asp-Tyr-Ser-Lys-Tyr-Leu-Asp~ ~Ser-Arg-Arg-Ala-Gln-Asp-Phe-Val-Gln-Trp-Leu-Met-Asn-Thr (or HSQGTFTSDYSKYLDSRRAQDFVQWLMNT) |
| Insulin (51) | Pancreatic Hormone (used in treatment of diabetes) |  |
The different amino acids that brand upwards a peptide or protein, and the society in which they are joined together past peptide bonds is referred to as the primary construction. From the examples shown above, information technology should be axiomatic that it is not a fiddling task to make up one's mind the principal structure of such compounds, even modestly sized ones.
Consummate hydrolysis of a protein or peptide, followed past amino acrid assay establishes its gross composition, but does not provide whatsoever bonding sequence information.
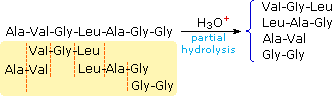
Partial hydrolysis will produce a mixture of shorter peptides and some amino acids. If the primary structures of these fragments are known, it is sometimes possible to deduce part or all of the original construction by taking advantage of overlapping pieces. For example, if a heptapeptide was composed of iii glycines, 2 alanines, a leucine and a valine, many possible chief structures could be written. On the other hand, if partial hydrolysis gave two known tripeptide and two known dipeptide fragments, as shown on the right, simple assay of the overlapping units identifies the original primary structure. Of course, this kind of structure determination is very inefficient and unreliable. Outset, we need to know the structures of all the overlapping fragments. Second, larger peptides would requite complex mixtures which would have to be separated and painstakingly examined to find suitable pieces for overlapping. It should be noted, however, that modern mass spectrometry uses this overlap technique effectively. The difference is that bond cleavage is not achieved by hydrolysis, and computers assume the time consuming chore of comparing a multitude of fragments.
3. N-Terminal Group Analysis
Over the years that chemists have been studying these important natural products, many techniques take been used to investigate their primary construction or amino acrid sequence. Indeed, commercial instruments that automatically sequence peptides and proteins are at present bachelor. A few of the near important and usually used techniques will be described here.
Identification of the North-terminal and C-terminal aminoacid units of a peptide concatenation provides helpful data. N-terminal analysis is accomplished past the Edman Degradation, which is outlined in the following diagram. A free amine function, normally in equilibrium with zwitterion species, is necessary for the initial bonding to the phenyl isothiocyanate reagent. The products of the Edman degradation are a thiohydantoin heterocycle incorporating the N-terminal amino acid together with a shortened peptide chain. Amine functions on a side-chain, as in lysine, may react with the isothiocyanate reagent, but do not requite thiohydantoin products.
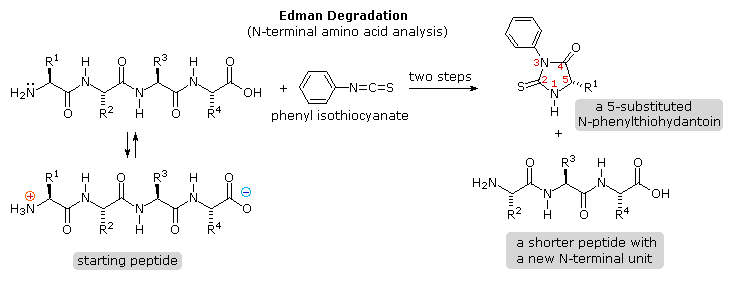 |
Repeated clicking of the " Adjacent Diagram " button displays the machinery of this of import analytical method.
A major reward of the Edman procedure is that the remaining peptide chain is not further degraded past the reaction. This means that the N-terminal analysis may be repeated several times, thus providing the sequence of the get-go three to 5 amino acids in the concatenation. A disadvantage of the procedure is that is peptides larger than 30 to 40 units practise non give reliable results.
4. C-Terminal Group Analysis
Chemic Analysis
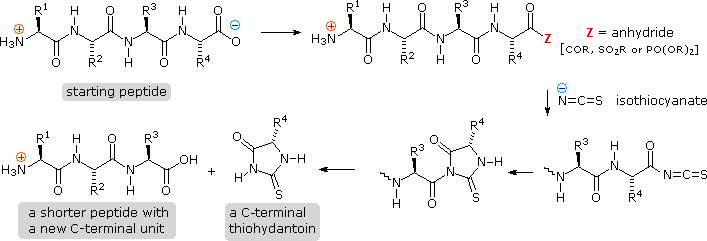
Complementary C-final analysis of peptide bondage may be accomplished chemically or enzymatically. The chemical analysis is slightly more than complex than the Edman procedure. First, side-chain carboxyl groups and hydroxyl groups must be protected equally amides or esters. Next, the C-terminal carboxyl group is activated as an anhydride and reacted with thiocyanate. The resulting acyl thiocyanate immediately cyclizes to a hydantoin ring, and this can be broken from the peptide chain in several ways, not described here. Depending on the nature of this final cleavage, the procedure can be modified to requite a C-concluding acyl thiocyanate peptide production which automatically rearranges to a thiohydantoin incorporating the penultimate C-terminal unit. Thus, repetitive analyses may be conducted in much the aforementioned way they are with the Edman procedure.
Enzymatic Analysis
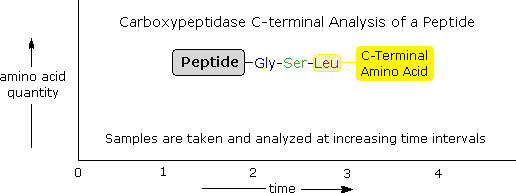
Enzymatic C-terminal amino acrid cleavage past i of several carboxypeptidase enzymes is a fast and convenient method of analysis. Because the shortened peptide product is also discipline to enzymatic cleavage, care must be taken to command the conditions of reaction so that the products of successive cleavages are properly monitored. The post-obit example illustrates this characteristic. A peptide having a C-final sequence: ~Gly-Ser-Leu is subjected to carboxypeptidase cleavage, and the complimentary aminoacids cleaved in this reaction are analyzed at increasing time intervals. By clicking on the diagram, the results of this experiment will be displayed. The leucine is cleaved showtime, the serine second, and the glycine third, as demonstrated past the sequential analysis. Of course, fourth and fifth units volition also exist released as fourth dimension passes, simply these products are non shown.
5. Selective Peptide Cleavage
| Name | Type | Specificity |
|---|---|---|
| Cyanogen Bromide | Chemical | Carboxyl Side of Methionine |
| Trypsin | Enzymatic | Carboxyl Side of Basic Amino Acids e.thousand. Lys & Arg |
| Chymotrypsin | Enzymatic | Carboxyl Side of Aryl Amino Acids e.thou. Phe, Tyr & Trp |
Mechanisms for the enzymatic reactions are not every bit easily formulated. Other enzymatic cleavages take been developed, but the two listed here will serve to illustrate their application.
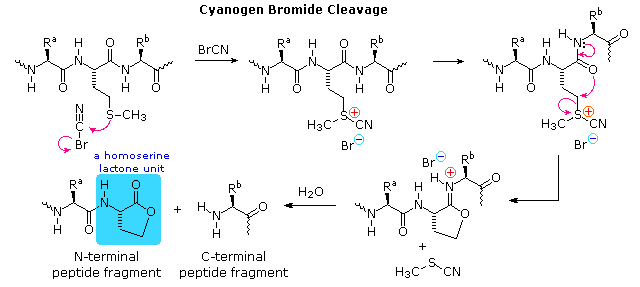
An Example of Principal Structure Analysis
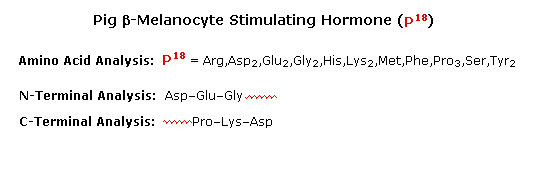
To see how these procedures can be combined to elucidate the primary structure of a peptide, consider the melanocyte stimulating hormone isolated from pigs. This octadecapeptide (18 amino acrid units) has the composition: Arg,Asp2,Glutwo,Glytwo,His,Lys2,Met,Phe,Pro3,Ser,Tyrii, and is abbreviated P18 . The post-obit diagram, which begins with the results of terminal unit analysis, illustrates the logical steps that could be used to solve the structural problem. By clicking the "Side by side Stage" push button the results and conclusions from each step will be displayed. Comments about each stage are presented under the diagram.
 |
a) Cyanogen bromide cleavage gives 2 peptide fragments, the longer of which has all the units on the C-terminal side of methionine.
b) N-last analysis of the undecapeptide fragment, P11 , locates the three amino acids to the correct of methionine.
c) Trypsin cleavage of Pxi shows the location of the unmarried arginine, which is institute as the C-terminal unit of the tetrapeptide fragment. Ane of the two lysines was known to be next to the C-terminus. The other must be part of the smaller peptide from the cyanogen bromide reaction.
d) With only four amino acids remaining to be located, the position of the 2nd tyrosine may be pursued by chymotrypsin cleavage of Pxviii itself. Four fragments are obtained, and the final structure might have been solved by these alone. Notwithstanding, selective terminal group analysis of the two pentapeptides serves to locate the tyrosine and a second proline next to the left virtually glycine, as well as identifying the units on each side of the methionine. The 1 remaining amino acid, a proline, is then placed at the last vacant site (yellow box).
vi. Cyclic Peptides
If the carboxyl office at the C-terminus of a peptide forms a peptide bail with the N-terminal amine grouping a cyclic peptide is formed. Carboxyate and amine functions on side chains may also combine to class rings. Cyclic peptides are almost commonly found in microorganisms, and oftentimes contain some D-amino acids equally well as unusual amino acids such as ornithine (Orn). The decapeptide antibiotic gramacidin S, produced by a strain of Bacillus brevis, is one example of this interesting class of natural products. The structure of gramicidin Due south is shown in the following diagram. The atypical amino acids are colored. When using a shorthand note for cyclic structures, the top line is written by the usual convention (Due north-group on the left), but vertical and lower lines must be adjusted to fit the bonding. Arrows on these bonds point in the CO-Due north management of each peptide bond.
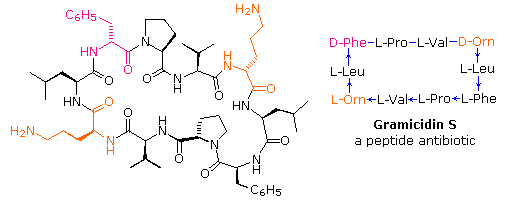
To meet a model of some other cyclic peptide, having potentially useful medicinal properties Click Here.
Structure-Property Relationships
The compounds we phone call proteins exhibit a broad range of physical and biological properties. Two general categories of unproblematic proteins are commonly recognized.
| Fibrous Proteins | As the name implies, these substances take fiber-like structures, and serve every bit the chief structural fabric in diverse tissues. Corresponding to this structural part, they are relatively insoluble in water and unaffected by moderate changes in temperature and pH. Subgroups within this category include: Collagens & Elastins, the proteins of connective tissues. tendons and ligaments. Keratins, proteins that are major components of skin, hair, feathers and horn. Fibrin, a protein formed when claret clots. | |
|---|---|---|
| Globular Proteins | Members of this course serve regulatory, maintenance and catalytic roles in living organisms. They include hormones, antibodies and enzymes. and either dissolve or form colloidal suspensions in water. Such proteins are more often than not more than sensitive to temperature and pH change than their fibrous counterparts. More Information |
one. The Secondary and Tertiary Construction of Large Peptides and Proteins
The various properties of peptides and proteins depend not only on their component amino acids and their bonding sequence in peptide bondage, but besides on the way in which the peptide chains are stretched, coiled and folded in infinite. Because of their size, the orientational options open to these macromolecules might seem virtually space. Fortunately, several factors act to narrow the structural options, and it is possible to identify some common structural themes or secondary structures that appear repeatedly in different molecules. These conformational segments are sometimes described by the dihedral angles Φ & Ψ, divers in the diagram on the right below. Most proteins and large peptides do not adopt completely uniform conformations, and full descriptions of their preferred three dimensional arrangements are defined as tertiary structures.
| Five factors that influence the conformational equilibria of peptide chains are: | 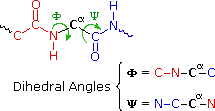 |
A. Helical Coiling
The relatively simple undecapeptide shown in the following diagram can adopt a zig-zag linear conformation, equally drawn. A ball & stick model of this peptide volition exist displayed past clicking the appropriate button. Even so, this molecule prefers to assume a coiled helical conformation, displayed by clicking any of the three buttons on the right. The middle push shows a stick model of this helix, with the backbone chain fatigued as a heavy black line and the hydrogen bonds as dashed maroon lines. The other buttons brandish a ball & stick model and a ribbon that defines this α-helix. Seven hydrogen bonds, that together provide roughly 30 kcal/mol stability, assistance to maintain this conformation.
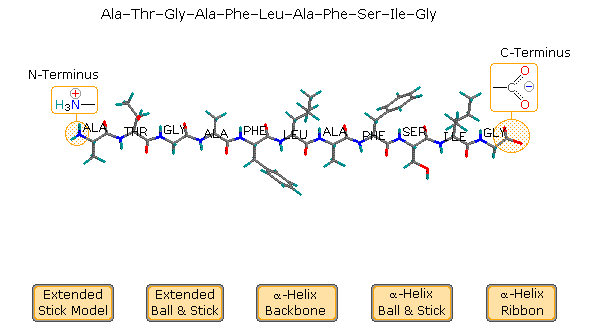
Examine the cartoon activated by the middle button. The N-terminal residuum (Ala) is on the left, and the C-terminal Gly on the right. The alpha-helix is right-handed, which ways that information technology rotates clockwise equally it spirals away from a viewer at either end. Other structural features that define an alpha-helix are: the relative locations of the donor and acceptor atoms of the hydrogen bond, the number of amino acid units per helical turn and the distance the turn occupies forth the helical axis. The first hydrogen bail (from the N-terminal terminate) is from the carbonyl group of the alanine to the N-H group of the phenylalanine. Three amino acids, Thr, Gly & Ala, fall entirely within this turn. Parts of the Due north-last alanine acceptor and the phenylalanine donor also fall inside this helical plough, and careful analysis of the structure indicates there are 3.6 amino acid units per turn. The distance covered past the turn is 5.4 Å. Using the dihedral angle terminology noted higher up, a perfect α-helix has Φ = -58º and Ψ = -47º. In natural proteins the values associated with α-helical conformations range from -57 to -70º for Φ, and from -35 to -48º for Ψ. To examine a model of this alpha-helix, click on the green circle. Once this display is activated, the important hormone insulin may exist shown past clicking the appropriate button in the blueish-shaded rows.
Helical conformations of peptide chains may as well be described by a two number term, nchiliad , where n is the number of amino acrid units per turn and m is the number of atoms in the smallest band divers by the hydrogen bail. Using this terminology, the alpha-helix is a 3.613 helix. Other common helical conformations are 310 and four.416. The alpha helix is the well-nigh stable of these, accounting for a third of the secondary structure institute in most globular (not-fibrous) proteins.
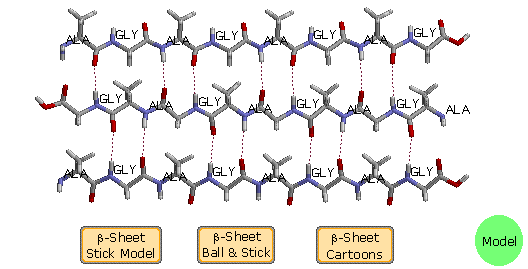
B. β-Pleated Sheets
The linear zig-zag conformation of a peptide chain may be stabilized by hydrogen bonding to adjacent parallel bondage of the same kind. Bulky side-chain substituents destabilize this arrangement due to steric crowding, and so this beta-sheet conformation is unremarkably limited to peptides having a big amount of glycine and alanine. Steric interactions also cause a slight bending or wrinkle of the peptide chains, and this results in a puckered distortion (the pleated sail). As shown in the post-obit diagram, the side by side chains may exist oriented in reverse Northward to C directions, termed antiparallel. Using the dihedral angle terminology, an antiparallel β-sheet has Φ = -139º and a Ψ = 135º. Alternatively, the next peptide chains may be oriented in the same management, termed parallel. By convention, beta-sheets are designated by broad arrows or cartoons, pointing in the direction of the C-terminus. In this diagram, these cartoons (colored violet) are displayed past clicking on the appropriate button. A model of a two-antiparallel-chain structure may be examined by clicking on the greenish circle.
Some proteins have layered stacks of β-sheets, which impart structural integrity and may open up to course a cavity (a beta barrel). An example is human retinol binding protein, which has a cavity formed by eight β-canvass strands. A model of this interesting protein may be displayed by clicking the upper push in the blue-shaded rows.
When beta-sheets are observed equally secondary structural components of globular proteins, they are twisted by about 5 to 25º per residue; consequently, the planes of the sheets are not parallel. The twist is always of the same handedness, and is usually greater for antiparallel sheets. Examples will exist constitute in the following structures.
C. Other Structures
Although most proteins and large peptides may have alpha-helix and beta-sail segments, their tertiary structures may consist of less highly organized turns, strands and coils. Turns reverse the direction of the peptide chain, and are considered to be a tertiary common secondary construction motif. Approximately a 3rd of all the residues in globular proteins are plant in turns. Turns occur importantly on the poly peptide surface, often incorporate polar and charged residues, and have been classified in three sub-groups.
Every bit noted before, several factors perturb the system of peptide chains. One that has not yet been cited is the structural influence of proline. Unlike the other common amino acids, rotation almost the α C-N bail in proline is non possible due to the structural constraint of the v-membered band. Consequently, the presence of a proline in a peptide chain introduces a curve or kink that disrupts helices or sheets. Too, prolines that are part of a peptide chain have no N-H hydrogen bonding donors to contribute to conformer stabilization.
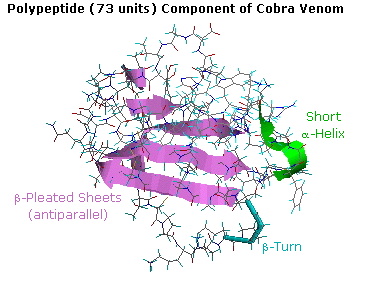
With the exception of silk fibroin and certain synthetically engineered peptides, meaning portions of well-nigh proteins adopt conformations that resist simple description or categorization. For example, the post-obit diagram shows the tertiary structure of a polypeptide neurotoxin found in cobra venom. A big section of antiparallel beta-sheets is colored violet, and a short alpha-helix is green. The remaining peptide chain seems disorganized, simply certain features such equally a 180º turn (chosen a beta-plow) and five disulfide bonds can be identified. A Chime model of this compound may be examined by clicking on the diagram.
Boosted Examples
A total description and word of protein structure is beyond the telescopic of this text, merely a few boosted examples will be instructive. In addition to the tertiary structures that volition exist displayed, attention must also be given to the way in which peptide structures may aggregate to form dimeric, trimeric and tetrameric clusters. These assemblies, known as quaternary structures, have characteristic properties different from their monomeric components. The examples of mellitin, collagen and hemoglobin, shown beneath demonstrate this characteristic.
Some proteins incorporate nonpeptide molecules in their overall structure, either bonded covalently or positioned by other forces. These are called conjugated proteins, and the non-peptide components are referred to as prosthetic groups. Examples of conjugated proteins include:
Glycoproteins, incorporating polysaccharide prosthetic groups (e.yard. collagen and mucus).
Lipoproteins, incorporating lipid prosthetic groups (eastward.g. HDL and LDL).
Chromoproteins, incorporating colored prosthetic groups (eastward.g. hemoglobin).
The seven illustrations shown beneath identify a set of peptides and proteins that may be examined every bit Jmol models by clicking on a selected picture.
Endothelin & Angiogenin are pocket-size peptides that have important and selective physiological properties.
Lysozyme a typical globular poly peptide, incorporating many identifiable secondary structures.
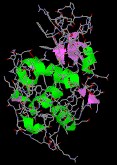
Mellitin, from honey bee venom, has a well-divers quaternary structure, half of which is shown hither.
Collagen is a widely distributed fibrous protein with a big and complex fourth construction. Only a small model segment is shown here.
Thioredoxin is a relatively small regulatory protein serving an of import redox function.
Hemoglobin, the most circuitous of these examples, is a large conjugated protein that transports oxygen. A 170 pound human being has about a kilogram of hemoglobin distributed among some five billion ruby claret cells. A liter of arterial blood at body temperature can send over 200 mL of oxygen, whereas the same fluid stripped of its hemoglobin will carry only 2 to 3 mL. The supramolecular associates of four subunits exemplifies a quaternary construction.
 | 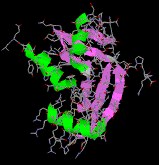 |  |  |
| endothelin | angiogenin | lysozyme | mellitin |
|---|
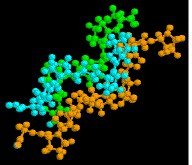 | 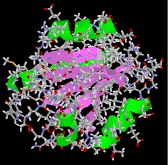 |  |
| collagen | thioredoxin | hemoglobin |
|---|
ii. Quaternary Structures of Proteins
Many proteins are actually assemblies of several polypeptides, which in the context of the larger aggregate are known every bit protein subunits. Such multiple-subunit proteins possess a fourth structure, in add-on to the third construction of the subunits. The subunits of a quaternary structure are held together by the same forces that are responsible for tertiary structure stabilization. These include hydrophobic attraction of nonpolar side chains in contact regions of the subunits, electrostatic interactions between ionic groups of reverse accuse: hydrogen bonds between polar groups; and disulfide bonds. Examples of proteins having a quaternary (or quartary) structure include hemoglobin, HIV-1 protease and the insulin hexamer.
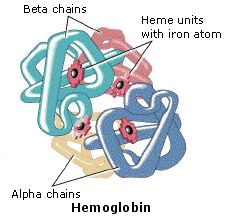 The hemoglobin molecule is an assembly of four protein subunits, two alpha units and two beta units. Each poly peptide concatenation folds into a set of alpha-helix structural segments connected together in a globin organization, and then called because this arrangement is the aforementioned folding motif used in other heme/globin proteins such as myoglobin. This folding pattern contains a pocket which strongly binds the heme group. The four polypeptide chains are leap to each other by common salt bridges, forming a tetrameric quaternary structure. A model of hemoglobin was shown above, and may also be examined by clicking the image on the left.
The hemoglobin molecule is an assembly of four protein subunits, two alpha units and two beta units. Each poly peptide concatenation folds into a set of alpha-helix structural segments connected together in a globin organization, and then called because this arrangement is the aforementioned folding motif used in other heme/globin proteins such as myoglobin. This folding pattern contains a pocket which strongly binds the heme group. The four polypeptide chains are leap to each other by common salt bridges, forming a tetrameric quaternary structure. A model of hemoglobin was shown above, and may also be examined by clicking the image on the left.
In animals, hemoglobin transports oxygen from the lungs or gills to the rest of the trunk, where it releases the oxygen for prison cell employ. Hemoglobin's oxygen-binding capacity is decreased in the presence of carbon monoxide because both gases compete for the aforementioned bounden sites on hemoglobin. The binding affinity of hemoglobin for CO is 200 times greater than its analogousness for oxygen. When hemoglobin combines with CO, information technology forms a very brilliant red compound chosen carboxyhemoglobin, which may cause the skin of CO poisoning victims to announced pink in death. In heavy smokers, up to 20% of the oxygen-active sites tin exist blocked past CO. Similarly, hemoglobin has a competitive binding affinity for cyanide, sulfur monoxide, nitrogen dioxide and sulfides including hydrogen sulfide . All of these bind to the heme iron without irresolute its oxidation land, causing grave toxicity.
Insulin is a peptide hormone composed of 51 amino acids, with a molecular weight of 5808 Da. Insulin has a strong result on metabolism and other body functions, causing cells in the liver, muscle, and fatty tissue to have up glucose from the blood, storing it as glycogen in the liver and muscle. Insulin is formed in the islets of Langerhans in the pancreas.
The molecular structure of insulin varies slightly between species of animals. Porcine (pig) insulin is peculiarly shut to the human version. Insulin molecules have a trend to form dimers in solution due to hydrogen-bonding between the C-termini of B bondage. In the presence of zinc ions, insulin dimers associate into hexamers. Insulin is stored in the trunk equally a hexamer, whereas the agile form is the monomer. These interactions have important clinical ramifications. Monomers and dimers readily lengthened into blood; hexamers diffuse poorly. By clicking the image on the far left, a model of the insulin monomer volition be displayed . A model of the hexamer will be shown by clicking its prototype.
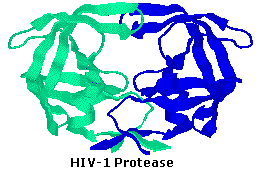 HIV-1 protease is an enzyme made past the HIV virus that is essential for it's life-bicycle. The virus makes sure proteins that demand to be cleaved or cut, in order to transform into functional proteins that enable the virus to infect new cells. HIV-1 protease cleaves the nascent proteins into their functional grade. The enzyme is composed of two symmetrically related subunits, shown hither in cartoon courage representation to highlight the secondary structure. Each subunit consists of the same pocket-sized chain of 99 amino acids, which come together in such as way every bit to form a tunnel where they meet. The protein to be cleaved sits in this tunnel, which houses the agile site of the enzyme. Two Asp-Thr-Gly catalytic triads, one on each chain, compose the active site. The two Asp's act as the main catalytic agents, and together with a water molecule cleave the protein chain bound in the tunnel. Without constructive HIV-1 protease, HIV virions remain uninfectious.
HIV-1 protease is an enzyme made past the HIV virus that is essential for it's life-bicycle. The virus makes sure proteins that demand to be cleaved or cut, in order to transform into functional proteins that enable the virus to infect new cells. HIV-1 protease cleaves the nascent proteins into their functional grade. The enzyme is composed of two symmetrically related subunits, shown hither in cartoon courage representation to highlight the secondary structure. Each subunit consists of the same pocket-sized chain of 99 amino acids, which come together in such as way every bit to form a tunnel where they meet. The protein to be cleaved sits in this tunnel, which houses the agile site of the enzyme. Two Asp-Thr-Gly catalytic triads, one on each chain, compose the active site. The two Asp's act as the main catalytic agents, and together with a water molecule cleave the protein chain bound in the tunnel. Without constructive HIV-1 protease, HIV virions remain uninfectious.
Because of its role in HIV replication, HIV-1 protease has been a target for antiviral drugs. Such drugs function as inhibitors, binding to the agile site by mimicking the tetrahedral intermediate of its substrate, thus disabling the enzyme. The construction of one such inhibitor, BEA388, will exist displayed on the left past clicking here.
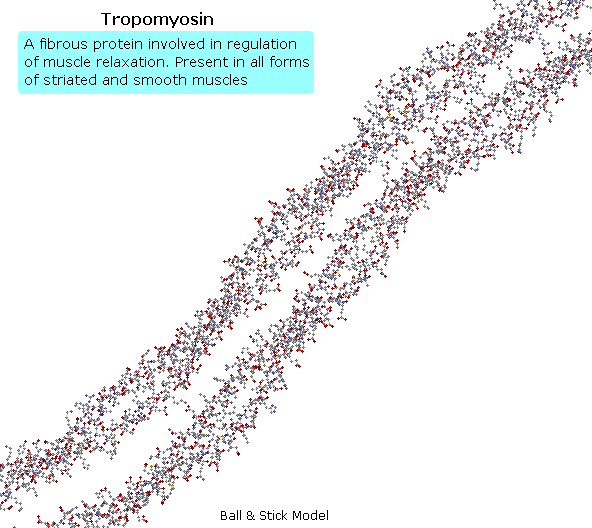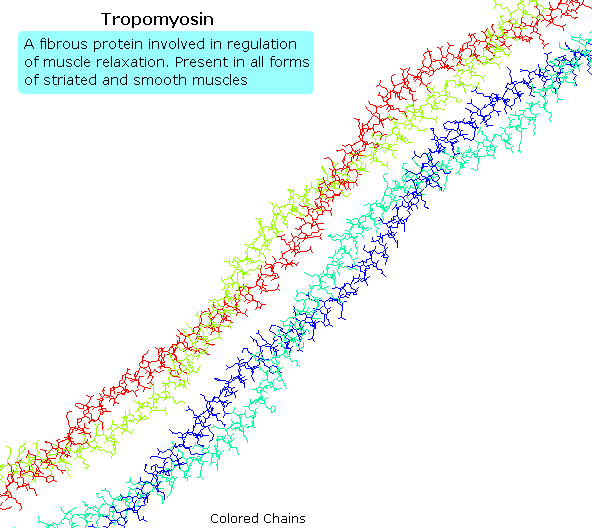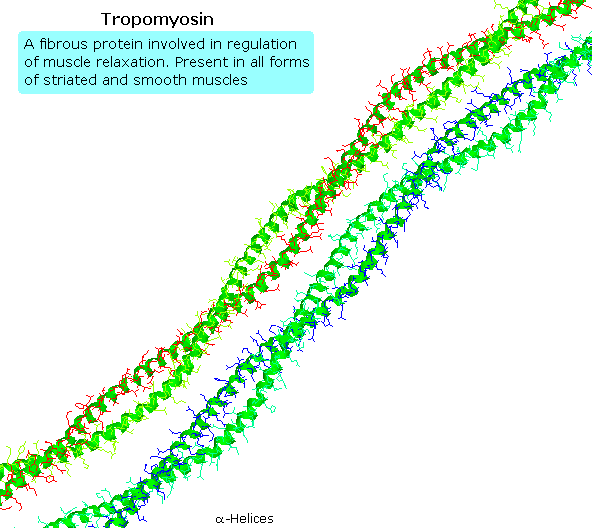
Tropomyosin
The following blitheness shows a segment of the fibrous protein tropomyosin, a common muscle regulator. The peptide chains are largely alpha-helices. These are wrapped in superhelix pairs, which are and then aligned in a parallel assortment.If animation is not occurring, click on the drawing or reload.
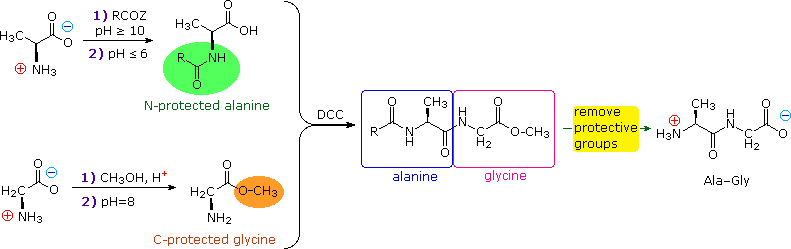
Peptide Synthesis
In order to synthesize a peptide from its component amino acids, ii obstacles must be overcome. The showtime of these is statistical in nature, and is illustrated by because the dipeptide Ala-Gly equally a proposed target. If we ignore the chemical science involved, a mixture of equal molar amounts of alanine and glycine would generate iv dissimilar dipeptides. These are: Ala-Ala, Gly-Gly, Ala-Gly & Gly-Ala. In the case of tripeptides, the number of possible products from these ii amino acids rises to eight. Conspicuously, some kind of selectivity must exist exercised if complex mixtures are to exist avoided.
The second difficulty arises from the fact that carboxylic acids and 1º or 2º-amines exercise not form amide bonds on mixing, only volition generally react past proton transfer to give salts (the intermolecular equivalent of zwitterion formation).
From the perspective of an organic chemist, peptide synthesis requires selective acylation of a free amine. To accomplish the desired amide bond germination, we must first deactivate all extraneous amine functions then they practice non compete for the acylation reagent. And so nosotros must selectively activate the designated carboxyl function so that it will acylate the one remaining free amine. Fortunately, chemic reactions that permit the states to accomplish these selections are well known.
First, the basicity and nucleophilicity of amines are substantially reduced by amide formation. Consequently, the acylation of amino acids by handling with acyl chlorides or anhydrides at pH > ten, as described earlier, serves to protect their amino groups from farther reaction.
Second, acyl halide or anhydride-similar activation of a specific carboxyl reactant must occur as a prelude to peptide (amide) bail formation. This is possible, provided competing reactions involving other carboxyl functions that might exist nowadays are precluded past preliminary ester formation. Call up, esters are weaker acylating reagents than either anhydrides or acyl halides, as noted before.
Finally, dicyclohexylcarbodiimide (DCC) effects the dehydration of a carboxylic acid and amine mixture to the corresponding amide nether relatively balmy conditions. The construction of this reagent and the machinery of its action have been described. Its application to peptide synthesis volition become apparent in the following discussion.
The strategy for peptide synthesis, every bit outlined here, should at present be credible. The following example shows a selective synthesis of the dipeptide Ala-Gly.
An important event remains to be addressed. Since the Northward-protective group is an amide, removal of this function might require conditions that would also cleave the only formed peptide bond. Furthermore, the harsh conditions oftentimes required for amide hydrolysis might crusade extensive racemization of the amino acids in the resulting peptide. This trouble strikes at the heart of our strategy, so it is important to requite conscientious idea to the pattern of specific Northward-protective groups. In item, 3 qualities are desired:
1) The protective amide should be easy to attach to amino acids.
2) The protected amino grouping should not react nether peptide forming conditions.
3) The protective amide group should exist easy to remove under mild atmospheric condition.
A number of protective groups that satisfy these weather have been devised; and 2 of the most widely used, carbobenzoxy (Cbz) and t-butoxycarbonyl (BOC or t-BOC), are described here.
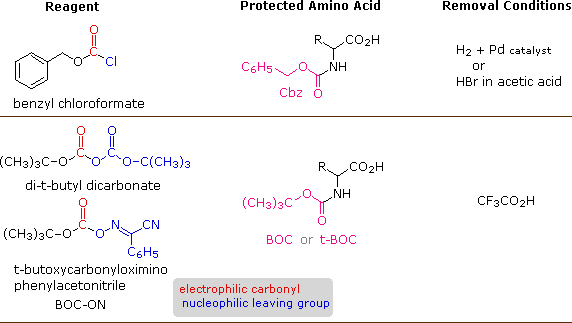
The reagents for introducing these Due north-protective groups are the acyl chlorides or anhydrides shown in the left portion of the higher up diagram. Reaction with a free amine function of an amino acid occurs speedily to give the "protected" amino acid derivative shown in the middle. This can and so be used to grade a peptide (amide) bond to a second amino acid. Once the desired peptide bail is created the protective group can exist removed under relatively mild non-hydrolytic conditions. Equations showing the protective group removal will be displayed above past clicking on the diagram. Cleavage of the reactive benzyl or tert-butyl groups generates a common carbamic acid intermediate (HOCO-NHR) which spontaneously loses carbon dioxide, giving the corresponding amine. If the methyl ester at the C-terminus is left in place, this sequence of reactions may be repeated, using a unlike Northward-protected amino acid equally the acylating reagent. Removal of the protective groups would and so yield a specific tripeptide, determined by the nature of the reactants and order of the reactions.
The synthesis of a peptide of significant length (e.g. ten residues) by this approach requires many steps, and the product must exist carefully purified subsequently each footstep to preclude unwanted cross-reactions. To facilitate the tedious and time consuming purifications, and reduce the material losses that occur in treatment, a clever modification of this strategy has been developed. This procedure, known equally the Merrifield Synthesis after its inventor R. Bruce Merrifield, involves attaching the C-terminus of the peptide chain to a polymeric solid, usually having the form of very small beads. Separation and purification is simply accomplished by filtering and washing the beads with appropriate solvents. The reagents for the adjacent peptide bond addition are then added, and the purification steps repeated. The entire procedure tin can be automated, and peptide synthesis machines based on the Merrifield arroyo are commercially available. A series of equations illustrating the Merrifield synthesis may be viewed by clicking on the following diagram. The last step, in which the completed peptide is released from the polymer back up, is a simple benzyl ester cleavage. This is not shown in the brandish.
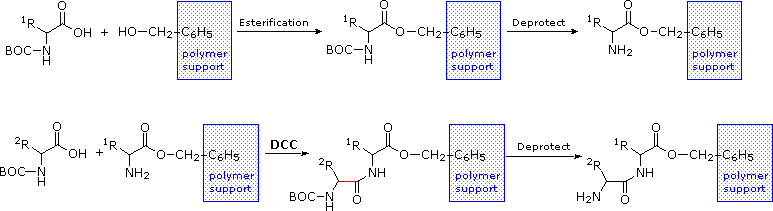 |
Two or more than moderately sized peptides can be joined together past selective peptide bond formation, provided side-chain functions are protected and do not interfere. In this mode good sized peptides and small proteins may be synthesized in the laboratory. However, even if chemists assemble the primary structure of a natural protein in this or any other manner, information technology may non immediately adopt its native secondary, 3rd and quaternary structure. Many factors, such as pH, temperature and inorganic ion concentration influence the conformational coiling of peptide chains. Indeed, scientists are still trying to sympathize how and why these higher structures are established in living organisms.
Denaturation
The natural or native structures of proteins may be altered, and their biological activity inverse or destroyed by treatment that does non disrupt the main structure. This denaturation is often done deliberately in the grade of separating and purifying proteins. For example, many soluble globular proteins precipitate if the pH of the solution is prepare at the pI of the poly peptide. Too, addition of trichloroacetic acrid or the bis-amide urea (NH2CONHii) is commonly used to effect protein precipitation. Following denaturation, some proteins will return to their native structures nether proper conditions; but farthermost conditions, such as potent heating, usually cause irreversible change.
Some treatments known to denature proteins are listed in the following table.
Denaturing Action | Mechanism of Operation |
|---|---|
| Heat | hydrogen bonds are broken by increased translational and vibrational free energy. (coagulation of egg white albumin on frying.) |
| Ultraviolet Radiation | Similar to heat (sunburn) |
| Potent Acids or Bases | salt formation; disruption of hydrogen bonds. (peel blisters and burns, protein precipitation.) |
| Urea Solution | competition for hydrogen bonds. (precipitation of soluble proteins.) |
| Some Organic Solvents | alter in dielectric constant and hydration of ionic groups. (disinfectant activity and precipitation of poly peptide.) |
| Agitation | shearing of hydrogen bonds. (beating egg white albumin into a meringue.) |
Not all proteins are easily denatured. Equally noted above, fibrous proteins such every bit keratins, collagens and elastins are robust, relatively insoluble, quaternary structured proteins that play important roles in the physical construction of organisms. Secondary structures such as the α-helix and β-sheet take on a dominant role in the architecture and aggregation of keratins. In addition to the intra- and intermolecular hydrogen bonds of these structures, keratins have large amounts of the sulfur-containing amino acid Cys, resulting in disulfide bridges that confer boosted strength and rigidity. The more flexible and elastic keratins of hair have fewer interchain disulfide bridges than the keratins in mammalian fingernails, hooves and claws. Keratins have a loftier proportion of the smallest amino acid, Gly, also as the side by side smallest, Ala. In the case of β-sheets, Gly allows sterically-unhindered hydrogen bonding between the amino and carboxyl groups of peptide bonds on side by side protein chains, facilitating their close alignment and strong binding. Fibrous keratin chains then twist effectually each other to form helical filaments.
Elastin, the connective tissue protein, also has a high percentage of both glycine and alanine. An insoluble rubber-like poly peptide, elastin confers elasticity on tissues and organs. Elastin is a macromolecular polymer formed from tropoelastin, its soluble precursor. The secondary structure is roughly 30% β-sheets, 20% α-helices and 50% unordered. The elastic properties of natural elastin are attributed to polypentapeptide sequences (Val-Pro-Gly-Val-Gly) in a cross-linked network of randomly coiled chains. Water is believed to act as a "plasticizer", assisting elasticity.
Collagen is a major component of the extracellular matrix that supports well-nigh tissues and gives cells construction. It has corking tensile force, and is the main component of fascia, cartilage, ligaments, tendons, bone and peel. Collagen contains more Gly (33%) and proline derivatives (xx to 24%) than exercise other proteins, just very trivial Cys. The main structure of collagen has a frequent repetitive design, Gly-Pro-X (where X is a hydroxyl bearing Pro or Lys). This kind of regular repetition and high glycine content is institute in just a few other fibrous proteins, such as silk fibroin (75-80% Gly and Ala + 10% Ser). Collagen bondage are approximately 1000 units long, and presume an extended left-handed helical conformation due to the influence of proline rings. Three such chains are wound about each other with a right-handed twist forming a rope-like superhelical fourth structure, stabilized by interchain hydrogen bonding.
Globular proteins are more soluble in aqueous solutions, and are generally more sensitive to temperature and pH change than are their fibrous counterparts; furthermore, they do not have the high glycine content or the repetitious sequences of the fibrous proteins. Globular proteins incorporate a variety of amino acids, many with big side chains and reactive functional groups. The interactions of these substituents, both polar and nonpolar, often causes the poly peptide to fold into spherical conformations which gives this class its name. In dissimilarity to the structural part played by the fibrous proteins, the globular proteins are chemically reactive, serving as enzymes (catalysts), transport agents and regulatory messengers.
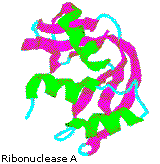 Although globular proteins are generally sensitive to denaturation (structural unfolding), some can be remarkably stable. I example is the small enzyme ribonuclease A, which serves to assimilate RNA in our food past cleaving the ribose phosphate bond. Ribonuclease A is remarkably stable. One process for purifying it involves treatment with a hot sulfuric acid solution, which denatures and partially decomposes most proteins other than ribonuclease A. This stability reflects the fact that this enzyme functions in the inhospitable environment of the digestive tract. Ribonuclease A was the starting time enzyme synthesized past R. Bruce Merrifield, demonstrating that biological molecules are simply chemical entities that may be constructed artificially.
Although globular proteins are generally sensitive to denaturation (structural unfolding), some can be remarkably stable. I example is the small enzyme ribonuclease A, which serves to assimilate RNA in our food past cleaving the ribose phosphate bond. Ribonuclease A is remarkably stable. One process for purifying it involves treatment with a hot sulfuric acid solution, which denatures and partially decomposes most proteins other than ribonuclease A. This stability reflects the fact that this enzyme functions in the inhospitable environment of the digestive tract. Ribonuclease A was the starting time enzyme synthesized past R. Bruce Merrifield, demonstrating that biological molecules are simply chemical entities that may be constructed artificially.
By clicking the cartoon image on the left, an interactive model of ribonuclease A volition be displayed.
| Render to Table of Contents |
|---|
This page is the property of William Reusch. Comments, questions and errors should be sent to whreusch@msu.edu.
These pages are provided to the IOCD to assist in chapters building in chemical education. 05/05/2013
![]()
Source: https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/protein2.htm
Posted by: crowellniae1979.blogspot.com


0 Response to "How To Draw A Peptide Bond"
Post a Comment